- Home
- Provider Administrative newsletter
- Response to ED boarding crisis, EDI changes, and regulatory guidance on abortion services in March 2023
Response to ED boarding crisis, EDI changes, and regulatory guidance on abortion services in March 2023
In this issue:
- Response to ED boarding crisis
- Rebranding–EDI Changes
- Regulatory guidance on abortion services and related care
- Update from Utilization Management
- Mass General Brigham Health Plan’s Drug fee schedules are to be updated
- Medical policy updates
- Code updates
- Formulary updates
Response to ED boarding crisis
Mass General Brigham Health Plan will comply with regulatory guidance to ensure hospitals are reimbursed for behavioral health crisis evaluations and stabilization services provided in the Emergency Department in response to the ED boarding crisis.
This will apply to:
- Commercial members, for dates of service 11/01/2022 and forward per MA DOI Bulletin 2022-08
- MassHealth members, for dates of service 01/03/2023 and forward per EOHHS guidance issued by the Office of Behavioral Health per MCE bulletin 93
Rebranding–EDI Changes
Now that we are officially Mass General Brigham Health Plan, we encourage providers to use our new name in all of their EDI submissions, especially on the claims (837) end. Please start using our new name: Mass General Brigham Health Plan.
In addition, if the Payer Name and Submitter Name on the claims file contains either NHP or AllWays Health Partners, please change that to MGBHP or Mass General Brigham Health Plan.
• On the 277 file sent to providers, we will replace NHP with MGBHP on the PR loop.
• The Claims Submission report will start reading MGBHP Claims submission report instead of NHP Claims submission report
Regulatory guidance on abortion services and related care
The Reproductive and Gender-Affirming Care Act (Chapter 127 of the Acts of 2022) became effective January 1, 2023. This new law and associated regulatory guidance provide legal protections for these services and mandate coverage for abortion and abortion-related care without cost-sharing for fully insured members upon renewal starting January 1, 2023. Qualified high-deductible health plans will continue to apply deductible cost-sharing consistent with federal rules for HSA-type plans. Please note, self-insured accounts and religious organizations that exclude abortion services can opt out of offering this cost-sharing change. In addition, these requirements do not add new mandates related to gender-affirming care.
For more information, please visit Mass.gov to review the regulatory guidance issued by the Division of Insurance.
Update from Utilization Management
1. For In-Network Providers: Use the "service end date" on the provider portal as the Next Review Date (NRD) to submit revision requests
- The service end date/NRD indicates when providers need to request additional days/units for inpatient stays
- Clinical must be included to support the requested units/days
- This does not apply to providers that have granted Mass General Brigham UM staff Electronic Medical Record (EMR) access
- If providers are interested in giving EMR access to the Mass General Brigham Utilization Management department team members, please email Mary-Kay Mykytyn at Mkmykytyn@MGB.org
2. If further portal assistance is needed, please outreach HealthPlanprweb@mgb.org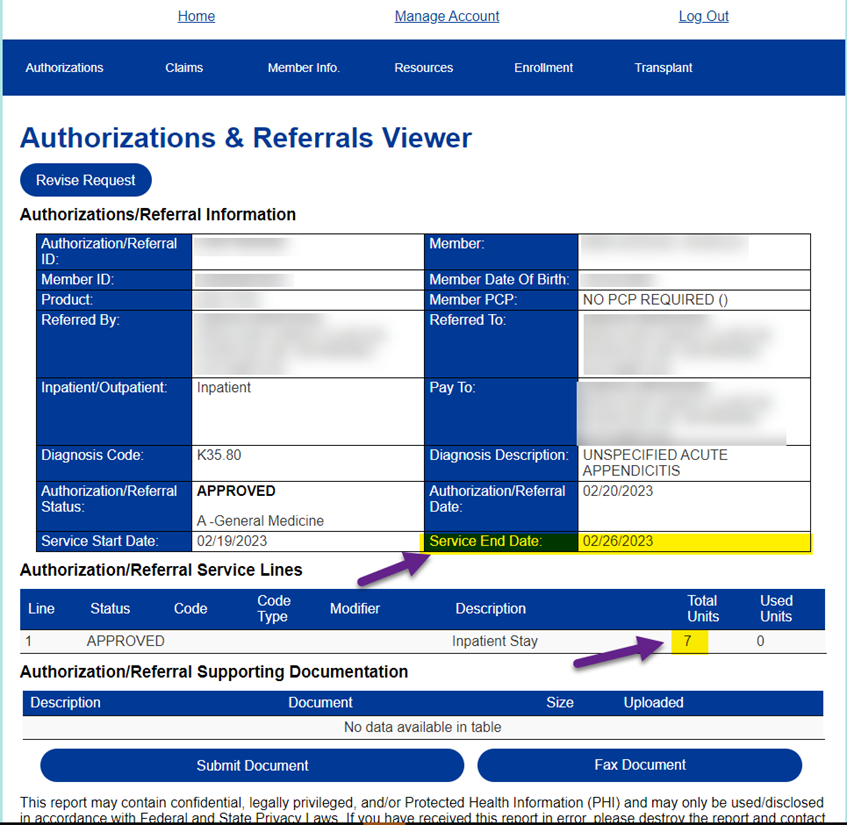
Mass General Brigham Health Plan’s Drug fee schedules are to be updated
Mass General Brigham Health Plan reviews its drug fee schedules quarterly to ensure that they are current, comprehensive and consistent with industry standards, to the extent supported by its systems. In most cases, changes involve adding fees for new or existing codes, to supplement the fees already on the fee schedule.
The next update will occur on April 1, 2023. Changes may involve both new and existing CPT and HCPCS codes, and will include the planned quarterly update to physician-administered drugs, immune globulin, vaccine and toxoid fees.
Medical policy updates
Five medical policies were reviewed and passed by the Mass General Brigham Health Plan’s Medical Policy Committee. These policies are now posted to MassGeneralBrighamHealthPlan.org. The table below is a summary.
For more information or to download our medical policies, go to https://www.massgeneralbrighamhealthplan.org/providers/medical-policies and select the policy under the medical policy listings.
|
Medical Policies |
|||
|
Policy Title |
Summary |
Products Affected |
Effective Date |
|
Continuous Glucose Monitors |
March 2023: Annual review. Prior authorization removed. |
All lines of business |
3/1/2023 |
|
Preimplantation Genetic Testing |
March 2023: Annual update. Medicare Advantage added to table. Subheading title changed to Preimplantation Genetic Testing (PGT). Exclusions updated. Medicare Advantage language added. Definitions updated |
All lines of business |
3/1/2023 |
|
Radiofrequency Ablation to Treat Uterine Fibroids |
March 2023: Annual review. Medicare Advantage added to table. References updated. |
All lines of business |
3/1/2023 |
|
Prostatic Urethral Lift |
Annual update. Medicare Advantage added to table. Medicare variation language added. References updated. |
All lines of business |
3/1/2023 |
|
Neuromodulation for Overactive Bladder and Fecal Incontinence |
Annual update. Medicare Advantage added to table. Medicare variation language added. References updated. |
All lines of business |
3/1/2023 |
In addition, Mass General Brigham Health Plan customized InterQual criteria and published a new guideline.
1. CP: Procedures. The guidelines are called Scar Revision (Custom) - MGB
To access this criteria, providers should log in to Mass General Brigham Health Plan’s provider website at MassGeneralBrighamHealthPlan.org and click the InterQual® Criteria Lookup link under the Resources Menu.
Code Updates
As a reminder to the network the following service(s) are not covered for all lines of business:
|
Code |
Description |
|
No Code |
Goniometer |
|
No Code |
Intense Pulse Light for Dry Eye Disease |
The following service(s) previously not covered will be covered with prior authorization for commercial and ASO lines of business only:
|
Code |
Description |
Effective Date |
|
0479T |
Fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children |
02/01/2023 |
|
0480T |
Fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof (List separately in addition to code for primary procedure) |
02/01/2023 |
|
0657T |
Vertebral body tethering, anterior; 8 or more vertebral segments |
02/01/2023 |
|
0421T |
Transurethral waterjet ablation of prostate, including control of post-operative bleeding, including ultrasound guidance, complete (vasectomy, meatotomy, cystourethroscopy, urethral calibration and/or dilation, and internal urethrotomy are included when performed) |
03/01/2023 |
The following service(s) will be covered with no prior authorization for commercial/ASO and ACO lines of business:
|
Code |
Description |
Effective Date |
|
78434 |
Absolute quantitation of myocardial blood flow (AQMBF), positron emission tomography (PET), rest and pharmacologic stress (List separately in addition to code for primary procedure) |
1/19/2023 |
|
C9482 |
Injection, sotalol HCl, 1 mg |
1/1/2016 |
|
A9276 |
Sensor; invasive (e.g., subcutaneous), disposable, for use with nondurable medical equipment interstitial continuous glucose monitoring system (CGM), one unit = 1 day supply |
3/1/2023 |
|
A9277 |
Transmitter; external, for use with nondurable medical equipment interstitial continuous glucose monitoring system (CGM) |
3/1/2023 |
|
A9278 |
Receiver (monitor); external, for use with nondurable medical equipment interstitial continuous glucose monitoring system (CGM) |
3/1/2023 |
|
A4238 |
Supply allowance for adjunctive, nonimplanted continuous glucose monitor (CGM), includes all supplies and accessories, 1 month supply = 1 unit of service |
3/1/2023 |
Drug Code Updates
The following drug(s) are now covered under the medical benefit with prior authorization for My Care Family plans:
|
Code |
Description |
Brand Name |
Effective Date |
|
J9042 |
Injection, brentuximab vedotin, 1 mg |
Adcetris |
4/1/2023 |
The following drug(s) are now covered under the medical benefit with prior authorization for Commercial and ASO lines of business:
|
Code |
Description |
Brand Name |
Effective Date |
|
No specific code |
Injection, pegfilgrastim-pbbk Prefilled syringe 6mg/0.6mL |
Fylnetra |
2/1/2023 |
|
No specific code |
Injection, eflapegrastim-xnst |
Rolvedon |
2/1/2023 |
|
No specific code |
Injection, pegfilgrastim-fpgk 6mg/0.6ML |
Stimufend |
2/1/2023 |
|
No specific code |
Injection, terlipressin 0.85mg |
Terlivaz |
2/1/2023 |
The following drug(s) are now covered under the medical benefit with prior authorization for Medicare Advantage lines of business:
|
Code |
Description |
Brand Name |
Effective Date |
|
No specific code |
Injection, pegfilgrastim-pbbk Prefilled syringe 6mg/0.6mL |
Fylnetra |
2/1/2023 |
|
No specific code |
Injection, pegfilgrastim-fpgk 6mg/0.6ML |
Stimufend |
2/1/2023 |
|
No specific code |
Injection, terlipressin 0.85mg |
Terlivaz |
2/1/2023 |
Formulary Updates
Effective 05/01/2023
DEFINITIONS
Formulary These drugs are included in AllWays Health Partners’ covered drug list.
Non-Formulary These drugs are not included in AllWays Health Partners’ formulary. AllWays Health Partners would only cover formulary alternatives. Providers can request Non-Formulary drugs as an exception, and AllWays Health Partners would require trial of all appropriate formulary alternatives prior to approving coverage of a Non-Formulary drug. If a Non-Formulary drug is approved, the member’s cost-sharing would be the highest tier.
Preferred These drugs are on AllWays Health Partners’ formulary and offer a lower cost to members.
Non-Preferred These drugs are on AllWays Health Partners’ formulary but offer a higher cost to members.
Excluded AllWays Health Partners does not cover these drugs. Members will receive a denial for all Excluded drug requests.
Updates for My Care Family Members
The following changes are being made to the listed medications:
MassHealth Unified Pharmacy Product List (UPPL) Updates:
|
Egrifta (tesamorelin) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Age requirement of ≥18 years of age and appropriate dosing/frequency · Initial approval duration was updated to 3 months and reauthorization approval duration was updated to 1 year |
|
Sunosi (solriamfetol) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Criteria will require results of sleep study, a treatment failure of cerebral stimulants and use in combination with other agents, and appropriate quantity · The quantity limits for Sunosi 75mg and 100mg were updated to 30 tablets per 30 days |
|
Xyrem, Xywav, Wakix |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Wakix - Requirement of treatment failures with a tricyclic antidepressant, SSRI, venlafaxine, or atomoxetine, and the indication of excessive daytime sleepiness (EDS) associated with narcolepsy was added · Xyrem, Xywav – treatment failure requirement with atomoxetine for diagnosis of cataplexy associated with narcolepsy · EDS associated with OSA was added to criteria as as off-label indication |
|
Modafinil, Armodafinil |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List) with the following quantity limits: · Modafinil 100mg, 45 tablets per 30 days · Modafinil 200mg, 60 tablets per 30 days · Armodafinil 50mg, 30 tablets per 30 days |
|
Hetlioz Hetlioz Liquid (tasimelteon) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Initial approval durations was updated to 12 months · Blindness was added as a requirement to the non-24hours sleep-wake disorder criteria |
|
Alimta pemetrexed |
These medications will no longer require a Prior Authorization under the medical benefit and are not available through the pharmacy benefit. |
|
Vivjoa (oteseconazole)
|
This medication will now require a prior authorization in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List) |
|
Monjuvi (tafasitamab-cxix) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). |
|
Arzerra (ofatumumab) Gazyva (obinutuzumab) Leukeran (chlorambucil) Zynlonta (loncastuximab) |
These medications will now require a prior authorization in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List).
Arzerra and Gazvya are available through the medical benefit only with a prior authorization. |
|
Kimmtrak (tebentafusp-tebn) |
This medication will now require a prior authorization in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List) |