- Home
- Provider Administrative newsletter
- Medicare Advantage training, Medicare balance, and prior authorization updates in January 2023
Medicare Advantage training, Medicare balance, and prior authorization updates in January 2023
In this issue
- Watch our Medicare Advantage training video
- Crisis Intervention – ED Boarding
- Medicare balance
- Medicare Advantage diagnosis codes
- Prior Authorization Lift for Inpatient Post-Acute Care (PAC)
- Hospital inpatient utilization report
- Mass Standard Oncology Chemo Form
- Early Intensive Behavioral Intervention (EIBI) providers
- Medical policy updates
- Formulary updates
- Reminders: Rebrand and Medicare Advantage
- HCPCS codes
- Code updates
- Drug code updates
Watch our Medicare Advantage training video
In this training, we'll cover:
- Medicare Advantage plan details
- The Medicare Advantage network
- Helpful resources
- What to expect next
Crisis Intervention – ED Boarding
Mass General Brigham Health Plan reimburses medical facilities for the provision of medically necessary, crisis intervention services to treat and stabilize to Mass General Brigham Health Plan members awaiting an inpatient acute psychiatric placement in a facility emergency department (ED) or observation setting. For more information, click here.
Medicare Balance
Medicare Balance is a state-regulated product that complements Medicare coverage or “wraps” around Medicare coverage by paying for the member’s Medicare deductibles and coinsurance minus any applicable plan copays. Also known as an “Indemnity Plan, ” members may access medical care from any provider who accepts Medicare payment.
Medicare Balance is a state-regulated product that complements Medicare coverage or “wraps” around Medicare coverage by paying for the member’s Medicare deductibles and coinsurance minus any applicable plan copays. Also known as an “Indemnity Plan, ” members may access medical care from any provider who accepts Medicare payment.
Key features:
-
No provider network
-
No primary care physician required
-
No referrals or prior authorizations required
-
Individual subscriber product (no family policies)
How Providers can submit claims for Medicare Balance:
-
Claims for Medicare Balance should be submitted to Medicare first
-
Medicare will process the primary claim and send payment to the provider
-
The Benefits Coordination and Recovery Center (BCRC) will then electronically “cross-over” the secondary claim to Mass General Brigham Health Plan
-
Mass General Brigham Health Plan will process the secondary claim and remit payment to the provider
-
Providers bill once and are paid twice. First by Medicare and then by Mass General Brigham Health Plan
-
Providers should not submit claims directly to Mass General Brigham Health Plan
For more info visit our claims page.
Medicare Advantage diagnosis codes
Beginning 1/1/2023, as a requirement for Medicare Advantage, providers will be required to submit a diagnosis code on any authorization requests submitted via the Provider Portal for Medicare Advantage members.
Prior Authorization Lift for Inpatient Post-Acute Care (PAC)
For dates of service 12/6/22 through 3/6/23 prior authorization review is suspended for initial review for members transferring from inpatient admissions at Acute Care hospitals, to Inpatient Post-Acute Care Facilities: skilled nursing facilities or acute rehab facilities. This does not include long-term or custodial admissions or homecare. Notifications should be submitted by the provider within 24 hours of admission and updates provided a minimum of every 5 days to enable Mass General Brigham Health Plan to support discharge planning. Concurrent review and retrospective review will proceed to determine the appropriateness of the level of care.
This applies to all lines of business as follows:
• 12/06/22-3/06/23: Commercial, Medicaid
• 1/1/23-3/06/23: Medicare Advantage (MA)
Hospital inpatient utilization report
The latest quarterly hospital inpatient utilization report is now available. To review this report, click on the Reports tab in the Provider Portal and select Clinical Reports. If you do not have access to the Provider Portal, you may register online at provider.massgeneralbrighamhealthplan.org.
Mass Standard Oncology Chemo Form
As of 12/1/22, AllWays Health Partners will be accepting the Mass Standard Oncology Chemo Form when requesting Oncology and Supportive care services. For more information, please visit DOI Bulletin 2022-07 (mass.gov). The form and other helpful Medical Specialty Pharmacy information can be found on our website.
Here is a link to the form: MASSACHUSETTS STANDARD FORM FOR CHEMOTHERAPY
Please note, as of February 1, 2023 this form will be required for all Oncology and Supportive care services. In addition, this form will be available electronically as of February 1, 2023.
Early Intensive Behavioral Intervention (EIBI) providers
Reminder: an important update for EIBI providers who see AllWays Health Partners members under 3 with an autism diagnosis who are also involved with Early Intervention.
What you need to know
- For dates of service starting 10/01/2022, EIBI providers must adopt the codes published by the Executive Office of Health and Human Services (EOHHS) in 101 CMR 358.00.
- AllWays Health Partners is updating prior authorization and claims systems to ensure EIBI providers can submit a prior authorization and receive accurate and timely claims payment based on the codes and rates published in 101 CMR 358.00.
|
EOHHS code mapping: |
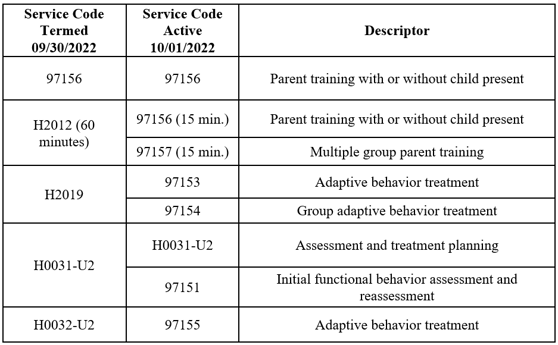 |
You can find the medical policy here and the payment guidelines here.
Rebrand reminder
AllWays Health Partners is now Mass General Brigham Health Plan as of January 1, 2023, to reflect and advance the system’s unique provider-payer integration that is improving health outcomes, reducing costs, and transforming the healthcare experience.
Please visit our Rebrand Provider FAQ for the latest information about our new name.
Medicare Advantage reminder
Under our new name, we are offering our first-ever Medicare Advantage products. This will give us the opportunity to support a growing population with a broad range of healthcare needs.
Please visit our Medicare Advantage Provider Page, as this will be updated on an ongoing basis with the latest information available.
Medical policy updates
Three medical policies were reviewed and passed by the Mass General Brigham Health Plan’s Medical Policy Committee. These policies are now posted to MassGeneralBrighamHealthPlan.org. The table below is a summary. For more information or to download our medical policies, go to massgeneralbrighamhealthplan.org/providers/medical-policies and select the policy under the medical policy listings.
|
Medical Policies |
|||
|
Policy Title |
Summary |
Products Affected |
Effective Date |
|
HIV-Associated Lipodystrophy Syndrome |
January 2023 Annual review. References updated. |
Commercial and Qualified Health Plans |
1/1/2023 |
|
Breast Surgeries |
January 2023: Annual Review. The following changes were made: • Under Coverage Guidelines; added clarifying statement about following MassHealth medical necessity criteria. • Under Breast Reconstruction Surgery; added coverage language to include nipple surgery/repair, and mastopexy. • Under Gender Affirming Procedures; added mastopexy. Added term “incongruence” to align with WPATH. • Under Breast Reconstruction Related to Other Medical Conditions; added amazia as additional medical condition. • Edited Nipple Repair subheading to include surgery. Added #2 under subheading. • Clarified exclusion #3 to include Gender Affirming Procedure. • References updated. |
Commercial and Qualified Health Plans |
1/1/2023 |
|
Insulin Pumps |
January 2023: Annual Review Under Overview section, added insulin pumps. Under Coverage Guidelines section, MassHealth statement section added. |
Commercial and Qualified Health Plans |
1/1/2023 |
In addition, as of January 2023, AllWays Health Partners is now Mass General Brigham Health Plan. As such, all customized InterQual criteria has been rebranded and you will see the new company name in the criteria.
To access this criteria, providers should log in to Mass General Brigham Health Plan’s provider website at MassGeneralBrighamHealthPlan.org and click the InterQual® Criteria Lookup link under the Resources Menu.
Formulary updates
Effective 03/01/2023
DEFINITIONS:
Formulary: These drugs are included in AllWays Health Partners’ covered drug list.
Non-Formulary: These drugs are not included in AllWays Health Partners’ formulary. AllWays Health Partners would only cover formulary alternatives. Providers can request Non-Formulary drugs as an exception, and AllWays Health Partners would require trial of all appropriate formulary alternatives prior to approving coverage of a Non-Formulary drug. If a Non-Formulary drug is approved, the member’s cost sharing would be the highest tier.
Preferred: These drugs are on AllWays Health Partners’ formulary and offer a lower cost to members.
Non-Preferred: These drugs are on AllWays Health Partners’ formulary but offer a higher cost to members.
Excluded: AllWays Health Partners does not cover these drugs. Members will receive a denial for all Excluded drug requests.
Updates for Commercial Members
The following changes are being made to the listed medications:
|
Nucala |
The following has been added to the pharmacy benefit with prior authorization: · Nucala prefilled syringe and injectable solution Both will remain on medical benefit as well.
Nucala auto-injector will remain on the pharmacy benefit only.
|
|
Fasenra |
The following has been added to the pharmacy benefit with prior authorization: · Fasenra prefilled syringe This will remain on medical benefit as well.
Fasenra Auto injectors will remain on the pharmacy benefit only |
|
Viibryd Starter Kit |
The following will no longer be considered formulary and will be non-formulary: · Viibryd Starter Kit
Generic Vilazodone is formulary and is available in the following strengths: · 10mg, 20mg, and 40mg. |
Updates for My Care Family Members
The following changes are being made to the listed medications:
MassHealth Unified Pharmacy Product List (UPPL) Updates
|
Breast Cancer Therapies |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Tykerb, Afinitor, Afinitor Disperz were added · Verbiage regarding combination therapy was updated throughout · Specific diagnoses are listed within criteria for each drug · Appendix “Requests which do not clearly document postmenopausal status” was removed |
|
Cerebral Stimulants & ADHD |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses are listed within criteria for each drug |
|
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulators |
Criteria was updated to include FDA-expanded age indication for Orkambi from ≤2 to ≤1 years of age.
New strengths and quantity limits (QL) were added for the following: · Orkambi 100-125mg tablet with QL 112 tablets per 28 days · Orkambi 75-94mg granule with QL 56 packets per 28 days · Orkambi 100-125mg granule with QL 56 packets per 28 days · Trikafta 50-25-37.5mg tablet with QL 84 tablets per 28 days |
|
Kinase Inhibitors |
Criteria for everolimus was updated to allow appropriate specialist for the requested indication, in place of just an oncology specialist. This applies to the following indications: · renal angiomyolipoma with tuberous sclerosis complex (TSC) · advanced pancreatic neuroendocrine tumors (PNET) · advanced neuroendocrine tumors (NET) of gastrointestinal or lung origin · subependymal giant cell astrocytoma (SEGA) with TSC
Step criteria of Inlyta and Keytruda combination for advanced RCC (clear cell histology) was removed from Cabometyx and Lenvima criteria.
Retevmo criteria was updated to include the expanded indication of adults with locally advanced or metastatic solid tumors with a rearranged during transfection (RET) gene fusion.
Ayvakit criteria was updated to require BOTH of the following: · Aggressive SM without the D816V c-Kit mutation or with c-Kit mutation status unknown + t/f with imatinib, and · D816V c-Kit mutation positive. Off-label indications are now included within criteria: · Koselugo for Plexiform Neurofibromatosis Type 1 ≥ 18 years of age · Nexavar for FLT3-ITD mutated AML The preferred drug status was removed from Inlyta and Sutent.
|
|
Lung Cancer Agents |
Portrazza, Rybrevant, and Zepzelca will now require a prior authorization in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). The following quantity limits were updated: Alunbrig 30 mg – 60 tablets per 30 days Lorbrena 25 mg – 30 tablets per 30 days
|
|
Adlarity (donepezil transdermal) |
This medication will now require a prior authorization in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List) with a quantity limit of 4 patches per 28 days.
|
|
Inhaled Respiratory Agents |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Gastroenterology was added as a specialty requirement for off-label utilization of budesonide for eosinophilic esophagitis · Off-label indication for Pulmicort suspension ≥ 13 years of age was included in criteria |
|
Xeljanz (tofacitinib citrate) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Removed the option for a low-cost alternative trial with traditional DMARDs for Xeljanz requests in PJIA and RA. Now will require only require treatment failure with an anti-TNF · Quantity limit for Xeljanz oral solution will now be 20 mL/day · Off-label indications: alopecia areata, HS, plaque psoriasis is included within criteria · Initial approval durations were clarified · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria · Strengths of 11 mg and 1 mg/mL solution were added to criteria
|
|
Cimzia (certolizumab pegol) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria · It was noted in all indications to bypass required biologic trials if a provider documents that Cimzia is preferred because the member is pregnant, breastfeeding or planning to become pregnant |
|
Otezla (apremilast) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria · Low-cost alternative trial requirement in PsA was removed · Off-label indications are now included within criteria |
|
Kineret, Skyrizi, Stelara, Humira, Enbrel, Cosentyx, Siliq, Kevzara, Ilumya, Tremfya |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria · Off-label indications are now included within criteria |
|
Rinvoq (upadacitinib) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria · Off-label indications are now included within criteria · Requirement of contraindication to BOTH Xeljanz and Xeljanz XR for psoriatic arthritis criteria · Removed the option for a low-cost alternative trial with traditional DMARDs for requests in RA and to specifically require an anti-TNF agent trial |
|
Infliximab Agents |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria · Off-label indications are now included within criteria · Added language regarding stability of requested medication for new members: Requests for Avsola and unbranded infliximab that document stability for any FDA-approved indication at an FDA-approved dose can be approved · Requests for Inflectra or Renflexis that document stability can be approved without documentation of failed trials with the preferred infliximab agents for ankylosing spondylitis or Crohn’s disease |
|
Olumiant (baricitinib) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria · Removed requirement of a trial with an anti-TNF agent for RA
|
|
Taltz (ixekizumab) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List) such as having specific diagnoses throughout the criteria. |
|
Simponi Simponi Aria Orencia |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria
|
|
Actemra (tocilizumab) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria · Language regarding stability of requested medication for new members with a documented history of hospitalization was added
|
|
Zeposia (ozanimod) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” throughout criteria · Removed requirement trial with Entyvio for Ulcerative Colitis |
|
Entyvio (vedolizumab) |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). Updates included the following: · Specific diagnoses have replaced “appropriate diagnosis” through criteria · Removed trial requirements from UC and CD criteria · Avsola was added as a trial requirement for fistulizing CD |
|
Anticonvulsants |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List) such as adding off-label indications within criteria. |
|
Chronic Myelogenous Leukemia Agents (CML) |
The preferred drug designation has been removed from Bosulif in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List). |
|
Lymphoma and Leukemia agents |
Criteria updated to include two off label indications (Venclexta for MM, Imbruvica for CNS Lymphoma). |
|
Melanoma agents |
Criteria was updated in order to be in compliance with the MassHealth UPPL (Unified Pharmacy Product List) such as adding off-label indications within criteria. |
|
COVID-19 OTC Testing Kits |
Criteria was updated to include CVS COVID-19 and Genabio testing kits with a quantity limit of 8 tests per 28 days. |
New January 2023 HCPCS codes
Not covered Experimental and Investigational:
|
C1747 |
Endoscope, single-use (i.e. disposable), urinary tract, imaging/illumination device (insertable) |
|
C1826 |
Generator, neurostimulator (implantable), includes closed feedback loop leads and all implantable components, with rechargeable battery and charging system |
|
C1827 |
Generator, neurostimulator (implantable), non-rechargeable, with implantable stimulation lead and external paired stimulation controller |
|
Q4262 |
Dual layer impax membrane, per square centimeter |
|
Q4263 |
Surgraft tl, per square centimeter |
|
Q4264 |
Cocoon membrane, per square centimeter |
Reportable Only; Not Reimbursable:
|
M0001 |
Advancing cancer care mips value pathways |
|
M0002 |
Optimal care for kidney health mips value pathways |
|
M0003 |
Optimal care for patients with episodic neurological conditions mips value pathways |
|
M0004 |
Supportive care for neurodegenerative conditions mips value pathways |
|
M0005 |
Promoting wellness mips value pathways |
|
M1150 |
Left ventricular ejection fraction (lvef) less than or equal to 40% or documentation of moderately or severely depressed left ventricular systolic function |
|
M1151 |
Patients with a history of heart transplant or with a left ventricular assist device (lvad) |
|
M1152 |
Patients with a history of heart transplant or with a left ventricular assist device (lvad) |
|
M1153 |
Patient with diagnosis of osteoporosis on date of encounter |
|
M1154 |
Hospice services provided to patient any time during the measurement period |
|
M1155 |
Patient had anaphylaxis due to the pneumococcal vaccine any time during or before the measurement period |
|
M1156 |
Patient received active chemotherapy any time during the measurement period |
|
M1157 |
Patient received bone marrow transplant any time during the measurement period |
|
M1158 |
Patient had history of immunocompromising conditions prior to or during the measurement period |
|
M1159 |
Hospice services provided to patient any time during the measurement period |
|
M1160 |
Patient had anaphylaxis due to the meningococcal vaccine any time on or before the patient's 13th birthday |
|
M1161 |
Patient had anaphylaxis due to the tetanus, diphtheria or pertussis vaccine any time on or before the patient's 13th birthday |
|
M1162 |
Patient had encephalitis due to the tetanus, diphtheria or pertussis vaccine any time on or before the patient's 13th birthday |
|
M1163 |
Patient had anaphylaxis due to the hpv vaccine any time on or before the patient's 13th birthday |
|
M1164 |
Patients with dementia any time during the patient's history through the end of the measurement period |
|
M1165 |
Patients who use hospice services any time during the measurement period |
|
M1166 |
Pathology report for tissue specimens produced from wide local excisions or re-excisions |
|
M1167 |
In hospice or using hospice services during the measurement period |
|
M1168 |
Patient received an influenza vaccine on or between July 1 of the year prior to the measurement period and june 30 of the measurement period |
|
M1169 |
Documentation of medical reason(s) for not administering influenza vaccine (e.g., prior anaphylaxis due to the influenza vaccine) |
|
M1170 |
Patient did not receive an influenza vaccine on or between July 1 of the year prior to the measurement period and june 30 of the measurement period |
|
M1171 |
Patient received at least one td vaccine or one tdap vaccine between nine years prior to the encounter and the end of the measurement period |
|
M1172 |
Documentation of medical reason(s) for not administering td or tdap vaccine (e.g., prior anaphylaxis due to the td or tdap vaccine or history of encephalopathy within seven days after a previous dose of a td-containing vaccine) |
|
M1173 |
Patient did not receive at least one td vaccine or one tdap vaccine between nine years prior to the encounter and the end of the measurement period |
|
M1174 |
Patient received at least one dose of the herpes zoster live vaccine or two doses of the herpes zoster recombinant vaccine (at least 28 days apart) anytime on or after the patient's 50th birthday before or during the measurement period |
|
M1175 |
Documentation of medical reason(s) for not administering zoster vaccine (e.g., prior anaphylaxis due to the zoster vaccine) |
|
M1176 |
Patient did not receive at least one dose of the herpes zoster live vaccine or two doses of the herpes zoster recombinant vaccine (at least 28 days apart) anytime on or after the patient's 50th birthday before or during the measurement period |
|
M1177 |
Patient received any pneumococcal conjugate or polysaccharide vaccine on or after their 60th birthday and before the end of the measurement period |
|
M1178 |
Documentation of medical reason(s) for not administering pneumococcal vaccine (e.g., prior anaphylaxis due to the pneumococcal vaccine) |
|
M1179 |
Patient did not receive any pneumococcal conjugate or polysaccharide vaccine, on or after their 60th birthday and before or during measurement period |
|
M1180 |
Patients on immune checkpoint inhibitor therapy |
|
M1181 |
Grade 2 or above diarrhea and/or grade 2 or above colitis |
|
M1182 |
Patients not eligible due to pre-existing inflammatory bowel disease (ibd) (e.g., ulcerative colitis, crohn's disease) |
|
M1183 |
Documentation of immune checkpoint inhibitor therapy held and corticosteroids or immunosuppressants prescribed or administered |
|
M1184 |
Documentation of medical reason(s) for not prescribing or administering corticosteroid or immunosuppressant treatment (e.g., allergy, intolerance, infectious etiology, pancreatic insufficiency, hyperthyroidism, prior bowel surgical interventions, celiac disease, receiving other medication, awaiting diagnostic workup results for alternative etiologies, other medical reasons/contraindication) |
|
M1185 |
Documentation of immune checkpoint inhibitor therapy not held and/or corticosteroids or immunosuppressants prescribed or administered was not performed, reason not given |
|
M1186 |
Patients who have an order for or are receiving hospice or palliative care |
|
M1187 |
Patients with a diagnosis of end stage renal disease (esrd) |
|
M1188 |
Patients with a diagnosis of chronic kidney disease (ckd) stage 5 |
|
M1189 |
Documentation of a kidney health evaluation defined by an estimated glomerular filtration rate (egfr) and urine albumin-creatinine ratio (uacr) performed |
|
M1190 |
Documentation of a kidney health evaluation was not performed or defined by an estimated glomerular filtration rate (egfr) and urine albumin-creatinine ratio (uacr) |
|
M1191 |
Hospice services provided to patient any time during the measurement period |
|
M1192 |
Patients with an existing diagnosis of squamous cell carcinoma of the esophagus |
|
M1193 |
Surgical pathology reports that contain impression or conclusion of or recommendation for testing of mmr by immunohistochemistry, msi by dna-based testing status, or both |
|
M1194 |
Documentation of medical reason(s) surgical pathology reports did not contain impression or conclusion of or recommendation for testing of mmr by immunohistochemistry, msi by dna-based testing status, or both tests were not included (e.g., patient will not be treated with checkpoint inhibitor therapy, no residual carcinoma is present in the sample [tissue exhausted or status post neoadjuvant treatment], insufficient tumor for testing) |
|
M1195 |
Surgical pathology reports that do not contain impression or conclusion of or recommendation for testing of mmr by immunohistochemistry, msi by dna-based testing status, or both, reason not given |
|
M1196 |
Initial (index visit) numeric rating scale (nrs), visual rating scale (vrs), or itchyquant assessment score of greater than or equal to 4 |
|
M1197 |
Itch severity assessment score is reduced by 2 or more points from the initial (index) assessment score to the follow-up visit score |
|
M1198 |
Itch severity assessment score was not reduced by at least 2 points from initial (index) score to the follow-up visit score or assessment was not completed during the follow-up encounter |
|
M1199 |
Patients receiving rrt |
|
M1200 |
Ace inhibitor (ace-i) or arb therapy prescribed during the measurement period |
|
M1201 |
Documentation of medical reason(s) for not prescribing ace inhibitor (ace-i) or arb therapy during the measurement period (e.g., pregnancy, history of angioedema to ace-i, other allergy to ace-i and arb, hyperkalemia or history of hyperkalemia while on ace-i or arb therapy, acute kidney injury due to ace-i or arb therapy), other medical reasons) |
|
M1202 |
Documentation of patient reason(s) for not prescribing ace inhibitor or arb therapy during the measurement period, (e.g., patient declined, other patient reasons) |
|
M1203 |
Ace inhibitor or arb therapy not prescribed during the measurement period, reason not given |
|
M1204 |
Initial (index visit) numeric rating scale (nrs), visual rating scale (vrs), or itchyquant assessment score of greater than or equal to 4 |
|
M1205 |
Itch severity assessment score is reduced by 2 or more points from the initial (index) assessment score to the follow-up visit score |
|
M1206 |
Itch severity assessment score was not reduced by at least 2 points from initial (index) score to the follow-up visit score or assessment was not completed during the follow-up encounter |
|
M1207 |
Number of patients screened for food insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety |
|
M1208 |
Number of patients not screened for food insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety |
|
M1209 |
At least two orders for high-risk medications from the same drug class, (table 4), not ordered |
|
M1210 |
At least two orders for high-risk medications from the same drug class, (table 4), not ordered |
Prior authorization required:
|
A4239 |
Supply allowance for non-adjunctive, non-implanted continuous glucose monitor (cgm), includes all supplies and accessories, 1 month supply = 1 unit of service |
|
C7504 |
Percutaneous vertebroplasties (bone biopsies included when performed), first cervicothoracic and any additional cervicothoracic or lumbosacral vertebral bodies, unilateral or bilateral injection, inclusive of all imaging guidance |
|
C7505 |
Percutaneous vertebroplasties (bone biopsies included when performed), first lumbosacral and any additional cervicothoracic or lumbosacral vertebral bodies, unilateral or bilateral injection, inclusive of all imaging guidance |
|
C7507 |
Percutaneous vertebral augmentations, first thoracic and any additional thoracic or lumbar vertebral bodies, including cavity creations (fracture reductions and bone biopsies included when performed) using mechanical device (eg, kyphoplasty), unilateral or bilateral cannulations, inclusive of all imaging guidance |
|
C7508 |
Percutaneous vertebral augmentations, first lumbar and any additional thoracic or lumbar vertebral bodies, including cavity creations (fracture reductions and bone biopsies included when performed) using mechanical device (eg, kyphoplasty), unilateral or bilateral cannulations, inclusive of all imaging guidance |
|
C9144C |
Injection, bupivacaine (posimir), 1 mg |
|
E2103 |
Non-adjunctive, non-implanted continuous glucose monitor or receiver |
|
G0330 |
Facility services for dental rehabilitation procedure(s) performed on a patient who requires monitored anesthesia (e.g., general, intravenous sedation (monitored anesthesia care) and use of an operating room |
|
J0225 |
Injection, vutrisiran, 1 mg |
|
J2021 |
Injection, linezolid (hospira) not therapeutically equivalent to j2020, 200 mg |
|
J2327 |
Injection, risankizumab-rzaa, intravenous, 1 mg |
|
J9046 |
Injection, bortezomib, (dr. reddy's), not therapeutically equivalent to j9041, 0.1 mg |
|
J9048 |
Injection, bortezomib (fresenius kabi), not therapeutically equivalent to j9041, 0.1 mg |
|
J9049 |
Injection, bortezomib (hospira), not therapeutically equivalent to j9041, 0.1 mg |
|
Q5126 |
Injection, bevacizumab-maly, biosimilar, (alymsys), 10 mg |
Prior authorization required for ACO plans; no prior authorization required for Commercial plans:
|
G0320 |
Home health services furnished using synchronous telemedicine rendered via a real-time two-way audio and video telecommunications system |
|
G0321 |
Home health services furnished using synchronous telemedicine rendered via telephone or other real-time interactive audio-only telecommunications system |
|
G0322 |
The collection of physiologic data digitally stored and/or transmitted by the patient to the home health agency (i.e., remote patient monitoring) |
Covered with no prior authorization required:
|
C7500 |
Debridement, bone including epidermis, dermis, subcutaneous tissue, muscle and/or fascia, if performed, first 20 sq cm or less with manual preparation and insertion of deep (eg, subfacial) drug-delivery device(s) |
|
C7501 |
Percutaneous breast biopsies using stereotactic guidance, with placement of breast localization device(s) (eg, clip, metallic pellet), when performed, and imaging of the biopsy specimen, when performed, all lesions unilateral and bilateral (for single lesion biopsy, use appropriate code) |
|
C7502 |
Percutaneous breast biopsies using magnetic resonance guidance, with placement of breast localization device(s) (eg, clip, metallic pellet), when performed, and imaging of the biopsy specimen, when performed, all lesions unilateral or bilateral (for single lesion biopsy, use appropriate code) |
|
C7503 |
Open biopsy or excision of deep cervical node(s) with intraoperative identification (eg, mapping) of sentinel lymph node(s) including injection of non-radioactive dye when performed |
|
C7506 |
Arthrodesis, interphalangeal joints, with or without internal fixation |
|
C7509 |
Bronchoscopy, rigid or flexible, diagnostic with cell washing(s) when performed, with computer-assisted image-guided navigation, including fluoroscopic guidance when performed |
|
C7510 |
Bronchoscopy, rigid or flexible, with bronchial alveolar lavage(s), with computer-assisted image-guided navigation, including fluoroscopic guidance when performed |
|
C7511 |
Bronchoscopy, rigid or flexible, with single or multiple bronchial or endobronchial biopsy(ies), single or multiple sites, with computer-assisted image-guided navigation, including fluoroscopic guidance when performed |
|
C7512 |
Bronchoscopy, rigid or flexible, with single or multiple bronchial or endobronchial biopsy(ies), single or multiple sites, with transendoscopic endobronchial ultrasound (ebus) during bronchoscopic diagnostic or therapeutic intervention(s) for peripheral lesion(s), including fluoroscopic guidance when performed |
|
C7513 |
Dialysis circuit, introduction of needle(s) and/or catheter(s), with diagnostic angiography of the dialysis circuit, including all direct puncture(s) and catheter placement(s), injection(s) of contrast, all necessary imaging from the arterial anastomosis and adjacent artery through entire venous outflow including the inferior or superior vena cava, fluoroscopic guidance, with transluminal balloon angioplasty of central dialysis segment, performed through dialysis circuit, including all required imaging, radiological supervision and interpretation, image documentation and report |
|
C7514 |
Dialysis circuit, introduction of needle(s) and/or catheter(s), with diagnostic angiography of the dialysis circuit, including all direct puncture(s) and catheter placement(s), injection(s) of contrast, all necessary imaging from the arterial anastomosis and adjacent artery through entire venous outflow including the inferior or superior vena cava, fluoroscopic guidance, with all angioplasty in the central dialysis segment, and transcatheter placement of intravascular stent(s), central dialysis segment, performed through dialysis circuit, including all required imaging, radiological supervision and interpretation, image documentation and report |
|
C7515 |
Dialysis circuit, introduction of needle(s) and/or catheter(s), with diagnostic angiography of the dialysis circuit, including all direct puncture(s) and catheter placement(s), injection(s) of contrast, all necessary imaging from the arterial anastomosis and adjacent artery through entire venous outflow including the inferior or superior vena cava, fluoroscopic guidance, with dialysis circuit permanent endovascular embolization or occlusion of main circuit or any accessory veins, including all required imaging, radiological supervision and interpretation, image documentation and report |
|
C7516 |
Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, with endoluminal imaging of initial coronary vessel or graft using intravascular ultrasound (ivus) or optical coherence tomography (oct) during diagnostic evaluation and/or therapeutic intervention including imaging supervision, interpretation and report |
|
C7517 |
Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, with iliac and/or femoral artery angiography, non-selective, bilateral or ipsilateral to catheter insertion, performed at the same time as cardiac catheterization and/or coronary angiography, includes positioning or placement of the catheter in the distal aorta or ipsilateral femoral or iliac artery, injection of dye, production of permanent images, and radiologic supervision and interpretation |
|
C7518 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) including intraprocedural injection(s) for bypass graft angiography with endoluminal imaging of initial coronary vessel or graft using intravascular ultrasound (ivus) or optical coherence tomography (oct) during diagnostic evaluation and/or therapeutic intervention including imaging, supervision, interpretation and report |
|
C7519 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) including intraprocedural injection(s) for bypass graft angiography with intravascular doppler velocity and/or pressure derived coronary flow reserve measurement (initial coronary vessel or graft) during coronary angiography including pharmacologically induced stress |
|
C7520 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) includes intraprocedural injection(s) for bypass graft angiography with iliac and/or femoral artery angiography, non-selective, bilateral or ipsilateral to catheter insertion, performed at the same time as cardiac catheterization and/or coronary angiography, includes positioning or placement of the catheter in the distal aorta or ipsilateral femoral or iliac artery, injection of dye, production of permanent images, and radiologic supervision and interpretation |
|
C7521 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography with right heart catheterization with endoluminal imaging of initial coronary vessel or graft using intravascular ultrasound (ivus) or optical coherence tomography (oct) during diagnostic evaluation and/or therapeutic intervention including imaging supervision, interpretation and report |
|
C7522 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation with right heart catheterization, with intravascular doppler velocity and/or pressure derived coronary flow reserve measurement (initial coronary vessel or graft) during coronary angiography including pharmacologically induced stress |
|
C7523 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, with endoluminal imaging of initial coronary vessel or graft using intravascular ultrasound (ivus) or optical coherence tomography (oct) during diagnostic evaluation and/or therapeutic intervention including imaging supervision, interpretation and report |
|
C7524 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, with intravascular doppler velocity and/or pressure derived coronary flow reserve measurement (initial coronary vessel or graft) during coronary angiography including pharmacologically induced stress |
|
C7525 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) with bypass graft angiography with endoluminal imaging of initial coronary vessel or graft using intravascular ultrasound (ivus) or optical coherence tomography (oct) during diagnostic evaluation and/or therapeutic intervention including imaging supervision, interpretation and report |
|
C7526 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) with bypass graft angiography with intravascular doppler velocity and/or pressure derived coronary flow reserve measurement (initial coronary vessel or graft) during coronary angiography including pharmacologically induced stress |
|
C7527 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with right and left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, with endoluminal imaging of initial coronary vessel or graft using intravascular ultrasound (ivus) or optical coherence tomography (oct) during diagnostic evaluation and/or therapeutic intervention including imaging supervision, interpretation and report |
|
C7528 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with right and left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, with intravascular doppler velocity and/or pressure derived coronary flow reserve measurement (initial coronary vessel or graft) during coronary angiography including pharmacologically induced stress |
|
C7529 |
Catheter placement in coronary artery(ies) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation, with right and left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) with bypass graft angiography with intravascular doppler velocity and/or pressure derived coronary flow reserve measurement (initial coronary vessel or graft) during coronary angiography including pharmacologically induced stress |
|
C7530 |
Dialysis circuit, introduction of needle(s) and/or catheter(s), with diagnostic angiography of the dialysis circuit, including all direct puncture(s) and catheter placement(s), injection(s) of contrast, all necessary imaging from the arterial anastomosis and adjacent artery through entire venous outflow including the inferior or superior vena cava, fluoroscopic guidance, with transluminal balloon angioplasty, peripheral dialysis segment, including all imaging and radiological supervision and interpretation necessary to perform the angioplasty and all angioplasty in the central dialysis segment, with transcatheter placement of intravascular stent(s), central dialysis segment, performed through dialysis circuit, including all imaging, radiological supervision and interpretation, documentation and report |
|
C7531 |
Revascularization, endovascular, open or percutaneous, femoral, popliteal artery(ies), unilateral, with transluminal angioplasty with intravascular ultrasound (initial noncoronary vessel) during diagnostic evaluation and/or therapeutic intervention, including radiological supervision and interpretation |
|
C7532 |
Transluminal balloon angioplasty (except lower extremity artery(ies) for occlusive disease, intracranial, coronary, pulmonary, or dialysis circuit), initial artery, open or percutaneous, including all imaging and radiological supervision and interpretation necessary to perform the angioplasty within the same artery, with intravascular ultrasound (initial noncoronary vessel) during diagnostic evaluation and/or therapeutic intervention, including radiological supervision and interpretation |
|
C7533 |
Percutaneous transluminal coronary angioplasty, single major coronary artery or branch with transcatheter placement of radiation delivery device for subsequent coronary intravascular brachytherapy |
|
C7534 |
Revascularization, endovascular, open or percutaneous, femoral, popliteal artery(ies), unilateral, with atherectomy, includes angioplasty within the same vessel, when performed with intravascular ultrasound (initial noncoronary vessel) during diagnostic evaluation and/or therapeutic intervention, including radiological supervision and interpretation |
|
C7535 |
Revascularization, endovascular, open or percutaneous, femoral, popliteal artery(ies), unilateral, with transluminal stent placement(s), includes angioplasty within the same vessel, when performed, with intravascular ultrasound (initial noncoronary vessel) during diagnostic evaluation and/or therapeutic intervention, including radiological supervision and interpretation |
|
C7537 |
Insertion of new or replacement of permanent pacemaker with atrial transvenous electrode(s), with insertion of pacing electrode, cardiac venous system, for left ventricular pacing, at time of insertion of implantable debribrillator or pacemake pulse generator (eg, for upgrade to dual chamber system) |
|
C7538 |
Insertion of new or replacement of permanent pacemaker with ventricular transvenous electrode(s), with insertion of pacing electrode, cardiac venous system, for left ventricular pacing, at time of insertion of implantable defibrillator or pacemaker pulse generator (eg, for upgrade to dual chamber system) |
|
C7539 |
Insertion of new or replacement of permanent pacemaker with atrial and ventricular transvenous electrode(s), with insertion of pacing electrode, cardiac venous system, for left ventricular pacing, at time of insertion of implantable defibrillator or pacemaker pulse generator (eg, for upgrade to dual chamber system) |
|
C7540 |
Removal of permanent pacemaker pulse generator with replacement of pacemaker pulse generator, dual lead system, with insertion of pacing electrode, cardiac venous system, for left ventricular pacing, at time of insertion of implantable defibrillator or pacemaker pulse generator (eg, for upgrade to dual chamber system) |
|
C7541 |
Diagnostic endoscopic retrograde cholangiopancreatography (ercp), including collection of specimen(s) by brushing or washing, when performed, with endoscopic cannulation of papilla with direct visualization of pancreatic/common bile ducts(s) |
|
C7542 |
Endoscopic retrograde cholangiopancreatography (ercp) with biopsy, single or multiple, with endoscopic cannulation of papilla with direct visualization of pancreatic/common bile ducts(s) |
|
C7543 |
Endoscopic retrograde cholangiopancreatography (ercp) with sphincterotomy/papillotomy, with endoscopic cannulation of papilla with direct visualization of pancreatic/common bile ducts(s) |
|
C7544 |
Endoscopic retrograde cholangiopancreatography (ercp) with removal of calculi/debris from biliary/pancreatic duct(s), with endoscopic cannulation of papilla with direct visualization of pancreatic/common bile ducts(s) |
|
C7545 |
Percutaneous exchange of biliary drainage catheter (eg, external, internal-external, or conversion of internal-external to external only), with removal of calculi/debris from biliary duct(s) and/or gallbladder, including destruction of calculi by any method (eg, mechanical, electrohydraulic, lithotripsy) when performed, including diagnostic cholangiography(ies) when performed, imaging guidance (eg, fluoroscopy), and all associated radiological supervision and interpretation |
|
C7546 |
Removal and replacement of externally accessible nephroureteral catheter (eg, external/internal stent) requiring fluoroscopic guidance, with ureteral stricture balloon dilation, including imaging guidance and all associated radiological supervision and interpretation |
|
C7547 |
Convert nephrostomy catheter to nephroureteral catheter, percutaneous via pre-existing nephrostomy tract, with ureteral stricture balloon dialation, including diagnostic nephrostogram and/or ureterogram when performed, imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation |
|
C7548 |
Exchange nephrostomy catheter, percutaneous, with ureteral stricture balloon dilation, including diagnostic nephrostogram and/or ureterogram when performed, imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation |
|
C7549 |
Change of ureterostomy tube or externally accessible ureteral stent via ileal conduit with ureteral stricture balloon dilation, including imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation |
|
C7550 |
Cystourethroscopy, with biopsy(ies) with adjuctive blue light cystoscopy with fluorescent imaging agent |
|
C7551 |
Excision of major peripheral nerve neuroma, except sciatic, with implantation of nerve end into bone or muscle |
|
C7552 |
Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) including intraprocedural injection(s) for bypass graft angiography and right heart catheterization with intravascular doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically induced stress, initial vessel |
|
C7553 |
Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with right and left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) with bypass graft angiography with pharmacologic agent administration (eg, inhaled nitric oxide, intravenous infusion of nitroprusside, dobutamine, milrinone, or other agent) including assessing hemodynamic measurements before, during, after and repeat pharmacologic agent administration, when performed |
|
C7554 |
Cystourethroscopy with adjunctive blue light cystoscopy with fluorescent imaging agent |
|
C7555 |
Thyroidectomy, total or complete with parathyroid autotransplantation |
|
C9143 |
Cocaine hydrochloride nasal solution (numbrino), 1 mg |
|
G0316 |
Prolonged hospital inpatient or observation care evaluation and management service(s) beyond the total time for the primary service (when the primary service has been selected using time on the date of the primary service); each additional 15 minutes by the physician or qualified healthcare professional, with or without direct patient contact (list separately in addition to cpt codes 99223, 99233, and 99236 for hospital inpatient or observation care evaluation and management services). (do not report g0316 on the same date of service as other prolonged services for evaluation and management 99358, 99359, 99418, 99415, 99416). (do not report g0316 for any time unit less than 15 minutes) |
|
G0317 |
Prolonged nursing facility evaluation and management service(s) beyond the total time for the primary service (when the primary service has been selected using time on the date of the primary service); each additional 15 minutes by the physician or qualified healthcare professional, with or without direct patient contact (list separately in addition to cpt codes 99306, 99310 for nursing facility evaluation and management services). (do not report g0317 on the same date of service as other prolonged services for evaluation and management 99358, 99359, 99418). (do not report g0317 for any time unit less than 15 minutes) |
|
G0318 |
Prolonged home or residence evaluation and management service(s) beyond the total time for the primary service (when the primary service has been selected using time on the date of the primary service); each additional 15 minutes by the physician or qualified healthcare professional, with or without direct patient contact (list separately in addition to cpt codes 99345, 99350 for home or residence evaluation and management services). (do not report g0318 on the same date of service as other prolonged services for evaluation and management 99358, 99359, 99417). (do not report g0318 for any time unit less than 15 minutes) |
|
G3002 |
Chronic pain management and treatment, monthly bundle including, diagnosis; assessment and monitoring; administration of a validated pain rating scale or tool; the development, implementation, revision, and/or maintenance of a person-centered care plan that includes strengths, goals, clinical needs, and desired outcomes; overall treatment management; facilitation and coordination of any necessary behavioral health treatment; medication management; pain and health literacy counseling; any necessary chronic pain related crisis care; and ongoing communication and care coordination between relevant practitioners furnishing care, e.g. physical therapy and occupational therapy, complementary and integrative approaches, and community-based care, as appropriate. required initial face-to-face visit at least 30 minutes provided by a physician or other qualified health professional; first 30 minutes personally provided by physician or other qualified health care professional, per calendar month. (when using g3002, 30 minutes must be met or exceeded.) |
|
G3003 |
Each additional 15 minutes of chronic pain management and treatment by a physician or other qualified health care professional, per calendar month. (list separately in addition to code for g3002. when using g3003, 15 minutes must be met or exceeded.) |
|
J0134 |
Injection, acetaminophen (fresenius kabi) not therapeutically equivalent to j0131, 10 mg |
|
J0136 |
Injection, acetaminophen (b braun) not therapeutically equivalent to j0131, 10 mg |
|
J0173 |
Injection, epinephrine (belcher) not therapeutically equivalent to j0171, 0.1 mg |
|
J0283 |
Injection, amiodarone hydrochloride (nexterone), 30 mg |
|
J0611 |
Injection, calcium gluconate (wg critical care), per 10 ml |
|
J0689 |
Injection, cefazolin sodium (baxter), not therapeutically equivalent to j0690, 500 mg |
|
J0701 |
Injection, cefepime hydrochloride (baxter), not therapeutically equivalent to maxipime, 500 mg |
|
J0703 |
Injection, cefepime hydrochloride (b braun), not therapeutically equivalent to maxipime, 500 mg |
|
J0877 |
Injection, daptomycin (hospira), not therapeutically equivalent to j0878, 1 mg |
|
J0891 |
Injection, argatroban (accord), not therapeutically equivalent to j0883, 1 mg (for non-esrd use) |
|
J0892 |
Injection, argatroban (accord), not therapeutically equivalent to j0884, 1 mg (for esrd on dialysis) |
|
J0893 |
Injection, decitabine (sun pharma) not therapeutically equivalent to j0894, 1 mg |
|
J0898 |
Injection, argatroban (auromedics), not therapeutically equivalent to j0883, 1 mg (for non-esrd use) |
|
J0899 |
Injection, argatroban (auromedics), not therapeutically equivalent to j0884, 1 mg (for esrd on dialysis) |
|
J1456 |
Injection, fosaprepitant (teva), not therapeutically equivalent to j1453, 1 mg |
|
J1574 |
Injection, ganciclovir sodium (exela) not therapeutically equivalent to j1570, 500 mg |
|
J1611 |
Injection, glucagon hydrochloride (fresenius kabi), not therapeutically equivalent to j1610, per 1 mg |
|
J1643 |
Injection, heparin sodium (pfizer), not therapeutically equivalent to j1644, per 1000 units |
|
J2184 |
Injection, meropenem (b. braun) not therapeutically equivalent to j2185, 100 mg |
|
J2247 |
Injection, micafungin sodium (par pharm) not thereapeutically equivalent to j2248, 1 mg |
|
J2251 |
Injection, midazolam hydrochloride (wg critical care) not therapeutically equivalent to j2250, per 1 mg |
|
J2272 |
Injection, morphine sulfate (fresenius kabi) not therapeutically equivalent to j2270, up to 10 mg |
|
J2281 |
Injection, moxifloxacin (fresenius kabi) not therapeutically equivalent to j2280, 100 mg |
|
J2401 |
Injection, chloroprocaine hydrochloride, per 1 mg |
|
J2402 |
Injection, chloroprocaine hydrochloride (clorotekal), per 1 mg |
|
J3244 |
Injection, tigecycline (accord) not therapeutically equivalent to j3243, 1 mg |
|
J3371 |
Injection, vancomycin hcl (mylan) not therapeutically equivalent to j3370, 500 mg |
|
J3372 |
Injection, vancomycin hcl (xellia) not therapeutically equivalent to j3370, 500 mg |
|
J9393 |
Injection, fulvestrant (teva) not therapeutically equivalent to j9395, 25 mg |
|
J9394 |
Injection, fulvestrant (fresenius kabi) not therapeutically equivalent to j9395, 25 mg |
Redirect to Optum:
|
C7900 |
Service for diagnosis, evaluation, or treatment of a mental health or substance use disorder, initial 15-29 minutes, provided remotely by hospital staff who are licensed to provide mental health services under applicable state law(s), when the patient is in their home, and there is no associated professional service |
|
C7901 |
Service for diagnosis, evaluation, or treatment of a mental health or substance use disorder, initial 30-60 minutes, provided remotely by hospital staff who are licensed to provided mental health services under applicable state law(s), when the patient is in their home, and there is no associated professional service |
|
C7902 |
Service for diagnosis, evaluation, or treatment of a mental health or substance use disorder, each additional 15 minutes, provided remotely by hospital staff who are licensed to provide mental health services under applicable state law(s), when the patient is in their home, and there is no associated professional service (list separately in addition to code for primary service) |
|
G0323 |
Care management services for behavioral health conditions, at least 20 minutes of clinical psychologist or clinical social worker time, per calendar month. (these services include the following required elements: initial assessment or follow-up monitoring, including the use of applicable validated rating scales; behavioral health care planning in relation to behavioral/psychiatric health problems, including revision for patients who are not progressing or whose status changes; facilitating and coordinating treatment such as psychotherapy, coordination with and/or referral to physicians and practitioners who are authorized by medicare to prescribe medications and furnish e/m services, counseling and/or psychiatric consultation; and continuity of care with a designated member of the care team) |
Redirect to Pharmacy:
|
J2311 |
Injection, naloxone hydrochloride (zimhi), 1 mg |
Code updates
The following service(s) previously not covered will be covered without prior authorization required for Commercial and ASO Plans:
|
Code |
Description |
Effective Date |
|
K1005 |
Disposable collection and storage bag for breast milk, any size, any type, each |
1/1/2023 |
The following service(s) will be covered with prior authorization via CareCentrix for all lines of business:
|
Code |
Description |
Effective Date |
|
95800 |
Sleep study, unattended, simultaneous recording; heart rate, oxygen saturation, respiratory analysis (eg, by airflow or peripheral arterial tone), and sleep time |
1/1/2023 |
The following service(s) will be covered without prior authorization for all lines of business:
|
Code |
Description |
Effective Date |
|
91316 |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, bivalent, preservative free, 10 mcg/0.2 mL dosage, for intramuscular use |
12/8/2022 |
|
0164A |
Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, bivalent, preservative free, 10 mcg/0.2 mL dosage, booster dose |
12/8/2022 |
|
91317 |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, bivalent spike protein, preservative free, 3 mcg/0.2 mL dosage, diluent reconstituted, tris-sucrose formulation, for intramuscular use |
12/8/2022 |
|
0173A |
Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, bivalent spike protein, preservative free, 3 mcg/0.2 mL dosage, diluent reconstituted, tris-sucrose formulation, third dose |
12/8/2022 |
Drug code updates
The following drug(s) are now covered under the medical benefit with prior authorization for MyCare Family Plans:
|
Code |
Description |
Brand Name |
Effective Date |
|
J1426 |
Injection, casimersen, 10 mg
|
Amondys 45 |
12/1/2022 |
|
J9039 |
Injection, blinatumomab, 1 mcg |
Blincyto |
1/1/2023 |
The following drug(s) are now covered under the medical benefit without prior authorization for Commercial and ASO Plans:
|
Code |
Description |
Brand Name |
Effective Date |
|
Q9991 |
Injection, buprenorphine extended-release (Sublocade), less than or equal to 100 mg |
Sublocade |
12/1/2022
|
|
Q9992 |
Injection, buprenorphine extended-release (Sublocade), greater than 100 mg |
The following drug(s) are now covered under the medical benefit with prior authorization for Commercial and ASO Plans:
|
Code |
Description |
Brand Name |
Effective Date |
|
No Specific code |
Injection, spesolimab-sbzo IV HCPCS J3590 can be billed to represent Spevigo 450/7.5 IV until such time CMS assigns a permanent code. |
Spevigo 450/7.5 |
12/1/2022 |
|
No Specific code |
Injection, olipudase alfa-rpcp IV HCPCS J3590 can be billed to represent Xenpozyme IV until such time CMS assigns a permanent code. |
Xenpozyme 20mg |
12/1/2022 |